First brain-chip patient from Neuralink plays online chess
Noland Arbaugh, 29, regained mobility below his shoulders after a post-diving accident with a Neuralink device, playing chess on a laptop and controlling the cursor
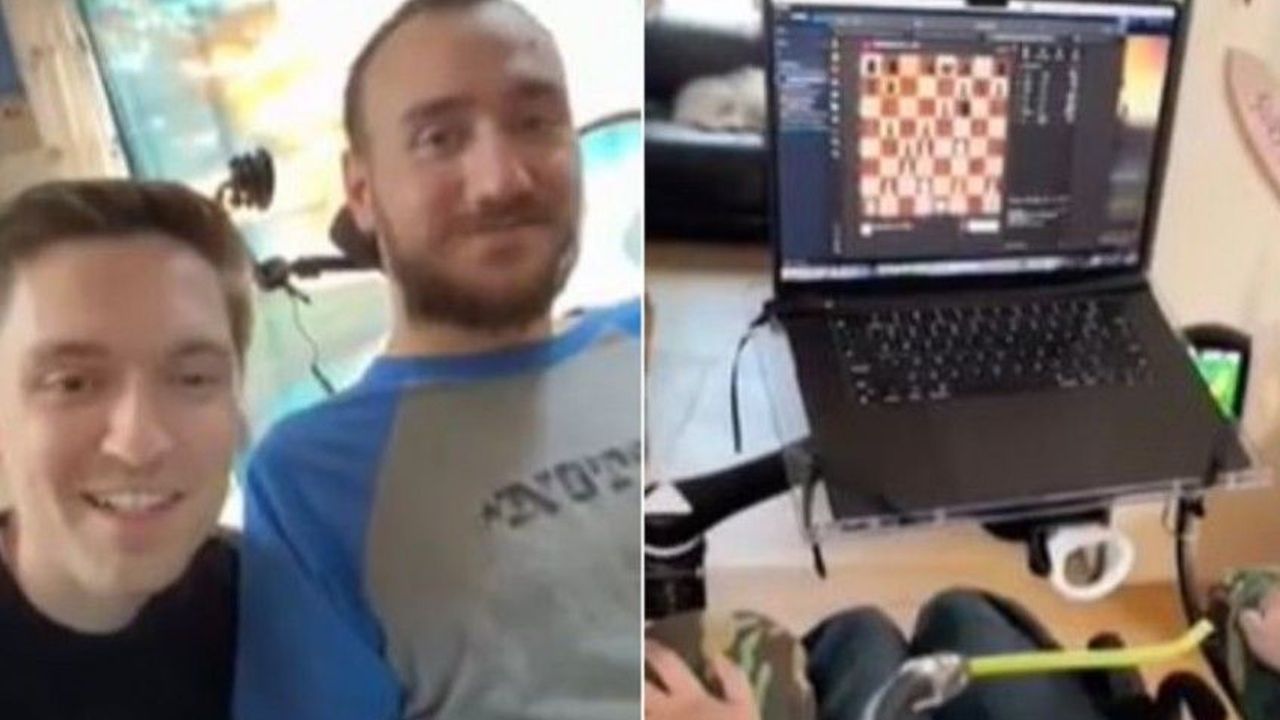
Elon Musk's company Neuralink, which specializes in brain-chip technology, conducted a live broadcast on March 20 showcasing its first patient who had been implanted with a chip that allowed him to use his thoughts to play chess online.
The patient, Noland Arbaugh, aged 29, had lost mobility below his shoulders due to a diving accident. With the help of the Neuralink device, he was able to play chess on his laptop and control the cursor.
The purpose of the implant is to empower individuals to operate a computer cursor or keyboard solely through their thoughts.
Arbaugh had received the implant in January and was able to control a computer mouse using his thoughts, as disclosed by Musk in February.
In a video shared on Musk's social media platform X, Arbaugh expressed that the surgery for the implant was straightforward, and he was discharged from the hospital the following day without any cognitive impairments.
Arbaugh mentioned that he had abandoned playing a game called Civilisation VI until Neuralink enabled him to resume playing for extended periods.
Reflecting on his interaction with the new technology, Arbaugh acknowledged that it is not flawless and that they have encountered some challenges.
He emphasized that there is still a long road ahead in perfecting the technology but highlighted the significant impact it has already had on his life.
Dr. Kip Ludwig, a former program director at the U.S. National Institutes of Health specializing in neural engineering, commented that Neuralink's demonstration was not groundbreaking. He noted that there is much to learn post-implantation to optimize the level of control achievable through the device.
Despite this, Dr. Ludwig recognized the positive step forward for patients in being able to interface with computers in ways previously impossible without the implant.
He described it as a promising initial phase in the advancement of this technology.
In February, Reuters reported that the US Food and Drug Administration (FDA) inspectors had identified issues with record-keeping and quality control in animal experiments at Neuralink, shortly after the company announced clearance to test its brain implants in humans.
Neuralink did not provide any responses to inquiries regarding the FDA's inspection at that time.
Source: Reuters







